Overview
The kidneys are paired retroperitoneal structures that are normally located between the transverse processes of T12-L3 vertebrae, with the left kidney typically somewhat more superior in position than the right. The upper poles are normally oriented more medially and posteriorly than the lower poles.
The kidneys serve important functions, including filtration and excretion of metabolic waste products (urea and ammonium); regulation of necessary electrolytes, fluid, and acid-base balance; and stimulation of red blood cell production. They also serve to regulate blood pressure via the renin-angiotensin-aldosterone system, controlling reabsorption of water and maintaining intravascular volume. The kidneys also reabsorb glucose and amino acids and have hormonal functions via erythropoietin, calcitriol, and vitamin D activation.
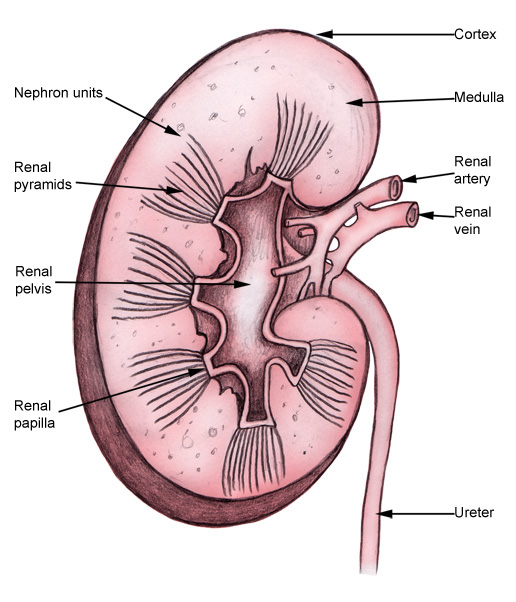
The kidney anatomy is shown in the image below
Gross Anatomy
Grossly, the kidneys are bean-shaped structures and weigh about 150 g in the male and about 135 g in the female. They are typically 10-12 cm in length, 5-7 cm in width, and 2-3 cm in thickness.
The relationship of neighboring organs to the kidneys is important, as described below:
- Superiorly, the suprarenal (adrenal) glands sit adjacent to the upper pole of each kidney
- On the right side, the second part of the duodenum (descending portion) abuts the medial aspect of the kidney
- On the left side, the greater curvature of the stomach can drape over the superomedial aspect of the kidney, and the tail of the pancreas may extend to overlie the renal hilum
- The spleen is located anterior to the upper pole and is connected by the splenorenal (lienorenal) ligaments
- Inferiorly to these organs, the colon typically rests anteriorly to the kidneys on both sides
- Posteriorly, the diaphragm covers the upper third of each kidney, with the 12th rib most commonly crossing the upper pole
- The kidneys sit over the psoas (medially) and the quadratus lumborum muscles (laterally)
- The images below further depict kidney anatomy and positioning
Vasculature
The kidneys receive approximately 20% of the cardiac output. The blood supply to the kidneys arises from the paired renal arteries at the level of L2. They enter into the renal hilum, the passageway into the kidney, with the renal vein anteriorly; the renal artery; and the renal pelvis posteriorly.The first branch off of the renal artery is the inferior suprarenal artery. The renal artery then branches off into 5 segmental branches. The posterior segmental artery supplies most of the posterior kidney, with the exception of the lower pole. The anterior branches are the superior segmental artery, anterior superior segmental artery, anterior inferior segmental artery, and inferior segmental artery. These arteries branch into interlobar arteries, which travel in a parallel fashion in between the major calyces and then branch further into arcuate arteries that run within the cortex across the bases of the renal pyramids.They then radiate into interlobular arteries, which extend into the cortex of the kidney to finally become afferent arterioles, then peritubular capillaries to efferent arterioles. Some of the terminal branches of the interlobular arteries become perforating radiate arteries, which supply the renal capsule. Renal pelvic and superior ureteric branches also originate from the renal artery and supply the upper portion of the collecting system (see the image below).The renal veins drain the kidneys in a similar distribution, and the renal vein is generally anterior to the renal artery at the hilum. The left renal vein is longer than the right as it crosses the midline to reach the inferior vena cava (IVC). Generally, the left gonadal vein drains into the left renal vein inferiorly, while the left suprarenal vein drains into the superior aspect of the renal vein at approximately the same level. Posteriorly, the left second lumbar vein typically drains into the left renal vein as well. The left renal vein then crosses under the origin of the superior mesenteric artery to reach the IVC. On the right side, the renal vein and gonadal vein drain separately and directly into the IVC.Renal lymphatics
The lymphatic drainage parallels the venous drainage system. After leaving the renal hilum, the left primary lymphatic drainage is into the left lateral aortic lymph nodes, including nodes anterior and posterior to the aorta between the inferior mesenteric artery and the diaphragm. On the right, it drains into the right lateral caval lymph nodes.Collecting system
Once the filtrate gets to the collecting ducts in the medulla of the kidney, they converge to a renal papilla, which represents the tip or apex of the renal pyramid. Urine then collects in typically 9-12 minor calyces, which then converge into 3-4 major calyces (significant variation is possible).The major calyces then empty into the renal pelvis, which passes urine through the ureteropelvic junction (UPJ) and into the ureter, which then propels urine distally to the bladder through peristalsis. The ureter may course posterior to the renal artery (or a lower pole branch) at its superior point, cross over the psoas muscle, and then pass posteriorly to the gonadal vessels. As it proceeds further distally, it passes over the iliac vessels and into the pelvis, finally traversing an intramural tunnel into the bladder and ending at the ureteral orifice on the trigone of the bladder (see the image below).Renal nerve anatomy/autonomic innervation
The kidney receives autonomic supply via both the sympathetic and parasympathetic portions of the nervous system. The preganglionic sympathetic nervous innervation to the kidneys arises from the spinal cord at the level of T8-L1. They synapse onto the celiac and aorticorenal ganglia and follow the plexus of nerves that run with the arteries. Activation of the sympathetic system causes vasoconstriction of the renal vessels. Parasympathetic innervation arises from the 10th cranial nerve (X), the vagus nerve, and causes vasodilation when stimulated.Microscopic Anatomy
The kidney is divided into the cortex and medulla. Renal pyramids in the medullary areas are separated by the cortical tissue called renal columns (of Bertin).Histology
The functional renal unit is the nephron, which is composed of the following:- The renal corpuscle: glomerulus and Bowman capsule
- Proximal convoluted tubules (PCT, located in the renal cortex)
- Descending loop of Henle (LOH)
- Ascending limb (which resides in the renal medulla, leading to the thick ascending limb)
- Thick ascending limb
- Distal convoluted tubule
- Collecting duct (which opens into the renal papilla)
Blood from the afferent glomerular arteriole passes through the juxtamedullary apparatus to the glomerulus. The glomerulus is a network of capillaries that filters blood across Bowman capsule into the proximal convoluted tubule.The glomerulus contains podocytes and a basement membrane allowing water and certain solutes to be filtered across. This filtrate then reaches the PCT, which reabsorbs glucose and various electrolytes along with water as the filtrate passes through. Meanwhile, after being filtered at the glomerulus, the blood passes into the efferent glomerular arteriole and then descends into the renal pyramid (see the images below).Natural Variants
Anatomic variations in the renal vasculature occur in approximately 25-40% of patients.[2]Supernumerary, or accessory, renal arteries are the most common arterial variation, with most of these branches supplying the lower pole of the kidney. They may pass anterior to the inferior vena cava (IVC) and over the ureteropelvic junction and be associated with (or cause) obstruction of the ureteropelvic junction (UPJ). Persistence of the right subcardinal vein anterior to the ureter can lead to a retrocaval ureter, which can also cause obstruction.Kidney position in the retroperitoneum is subject to variation as well. A kidney may be in an ectopic location, such as the pelvis, when it doesn’t ascend properly, or it can be malrotated or fused (as in horseshoe kidneys, in which the inferior poles are fused, causing a U-shaped configuration).In some fusion anomalies, such as crossed-fused ectopia, the 2 kidneys may be located on the same side. Although some of these variations may be associated with pathological conditions, such as hydronephrosis and UPJ obstruction, they can also remain completely asymptomatic and undiscovered until a diagnosis is made by radiographic study.Importantly, in an ectopic kidney, the adrenals should still be in the superior portion of the posterior peritoneum, since their embryologic origin is different from that of the kidneys.Variants may also exist in the collecting system drainage. Duplication anomalies may develop, wherein more than a single collecting system may form and drain separately into the bladder (complete duplication) or join at some point proximally before draining into a single orifice into the urinary bladder (partial duplication). In a complete duplicated system, the upper pole moiety drains inferomedially into the bladder, and the lower pole moiety drains superolaterally, as described by the Weigert-Meyer rule.Pathophysiological Variants
Crossing vessel, UPJ obstruction, vesicoureteral reflux
Crossing vessel, ureteropelvic junction (UPJ) obstruction, or vesicoureteral reflux can become pathophysiologic if it causes extrinsic or primary intrinsic obstruction leading to hydronephrosis. This can be seen with aberrant crossing vessels in a single system, which leads to UPJ obstruction. Obstruction can also occur from an ectopic ureter, where it is commonly seen inserting inferomedially in an abnormal location (ie, bladder neck) and is often associated with the upper pole moiety of a complete duplicated collecting system.Similarly, a ureterocele in a single system, or sometimes seen in a complete duplicated system, can cause obstruction. From an intrinsic standpoint, UPJO can also be caused by an adynamic/aperistaltic segment of ureter that is due to abnormal embryologic development. Secondary etiologies of obstruction include stones, infections, iatrogenic ureteral damage causing strictures, and other acquired factors that are not due to anatomic variants.Vesicoureteral reflux is another variation and is caused by an abnormal insertion of the ureter in the bladder in an abnormal position (usually superolateral). This insertion site leads to a shorter intramural tunnel length for the ureter to pass through the bladder wall, which leads to inadequate compression of the ureter during bladder filling and contraction and may allow reflux of urine up the ureter. Vesicoureteral reflux can contribute to pyelonephritis and, in extreme situations, irreversible damage to an affected renal unit.Surgical Considerations
Surgical approach
Extraperitoneal flankWhen considering surgical approaches to the kidney, understanding the benefits, risks, and complications of each approach is paramount. The kidneys are retroperitoneal organs and, therefore, during an open surgery, one may choose to use an extraperitoneal flank approach, incising over the 11th-12th rib, plus or minus rib excision and staying retroperitoneally. This is particularly useful in obese patients, but not in patients with severe scoliosis or cardiorespiratory disorders, and provides direct access to the retroperitoneal space without traversing the peritoneal cavity.Dorsal lumbotomyIn the dorsal lumbotomy approach (which currently is rarely used in urologic surgery except in pediatric pyeloplasties for ureteropelvic junction repair), the patient is placed in a prone position, and a smaller muscle-sparing incision is made posteriorly without rib resection. It also provides a direct, retroperitoneal approach with a smaller incision but may be technically limiting in surgical exposure in larger patients and with differing pathology.Transabdominal approachThe transabdominal approach is performed with the patient lying supine and making either a subcostal or midline incision to traverse the peritoneum or reach the retroperitoneum. The renal pedicle is approached more directly using this technique, which is ideally suited to large and complex tumors or trauma, as the vessels can be approached quite proximally to establish early vascular control. Potential complications of the approach include postoperative ileus and future adhesion formation.Thoracoabdominal approachThe thoracoabdominal approach involves a lengthy incision over the chest wall and abdomen over the operative site. This approach is most often used for large tumors in radical nephrectomy, especially for those in the upper pole. Its disadvantages are increased pain and longer hospital stay and convalescence, as well as potential injury to the parietal pleura necessitating a thoracostomy tube.[6]Laparoscopic approachFrom a minimally invasive perspective, laparoscopic and robotic-assisted laparoscopic approaches have been used for nearly all renal procedures, typically with reduced morbidity (primarily decreased postoperative pain and faster return to normal activity) when compared with open surgical techniques. Both transperitoneal and direct retroperitoneal approaches have been used and well described in the literature, and each has benefits and drawbacks to consider.Percutaneous approachIn percutaneous surgery, defining the calyceal anatomy is key. Patients are placed most commonly in the prone position, and direct entry into/through the retroperitoneal space allows access to the kidneys. For percutaneous stone surgery, access into the collecting system is performed under radiologic guidance. In other percutaneous procedures, such as thermoablation of small renal tumors, direct access to the site of pathology under real-time ultrasound or CT scan allows image-guided biopsy and treatment of lesions.












0 التعليقات:
إرسال تعليق